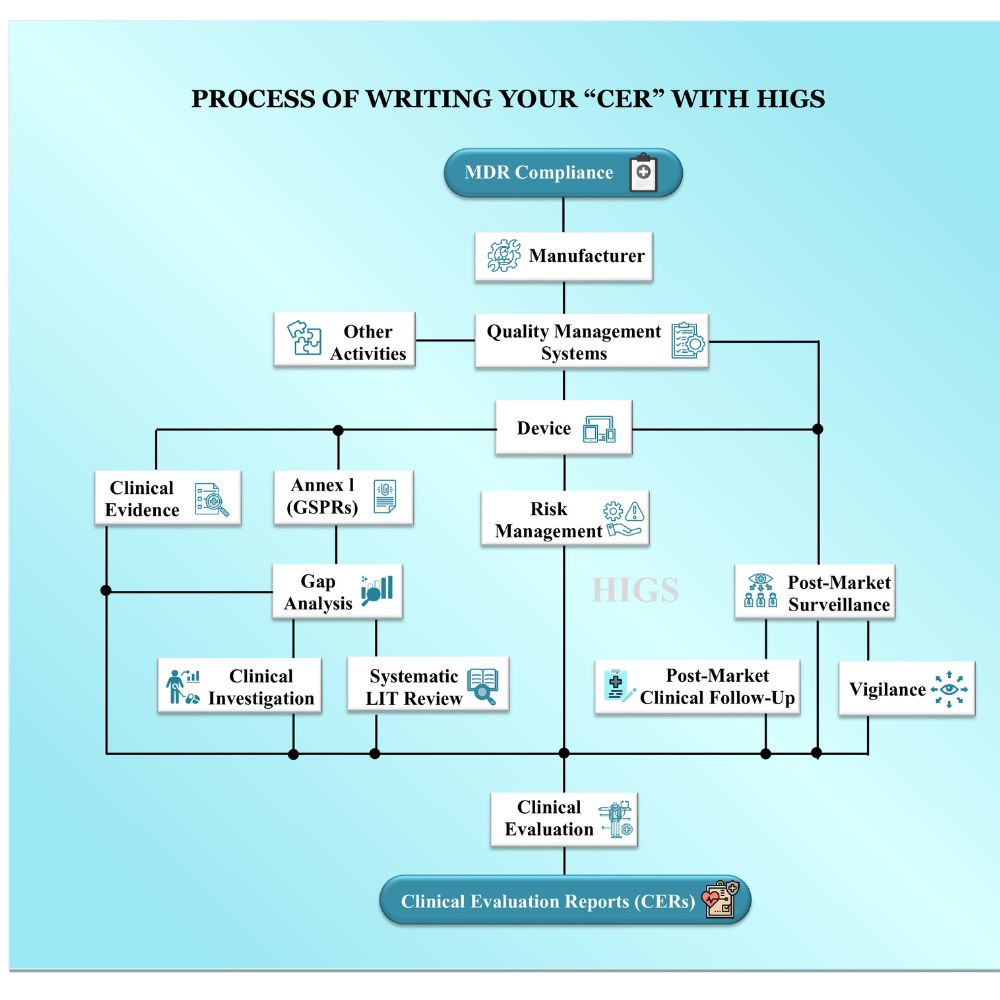
Hi there, welcome to our site, we are your complete phd guidance spot, where you can expect a high quality work. Your work will be secured since we neither have tie-up with any university nor
outsource your work. We don't share your details with other clients. The person next to you might also be our client, but you may not know... we are that confidential. And our employees are full-time
workers.